The smartest way
to dossier.
From preclinical IND to postmarket, Weave transforms every stage of therapeutic development.
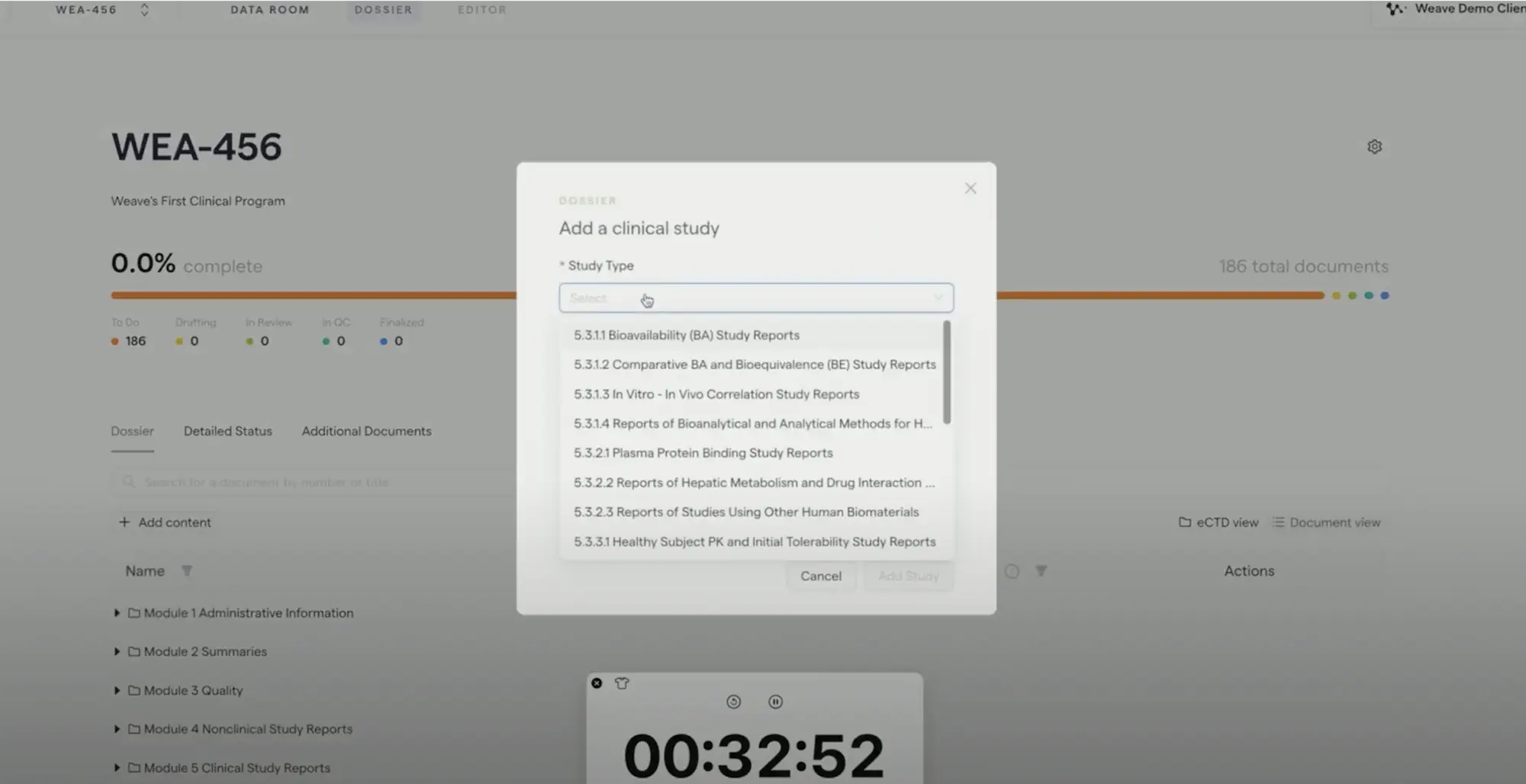
How it works
Upload
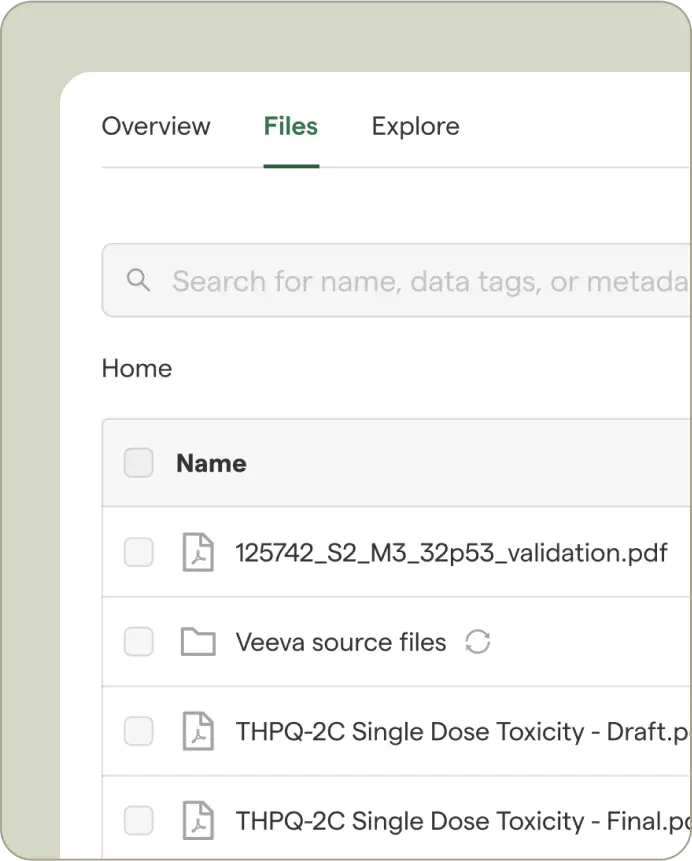
Start with your source data and select from customizable templates aligned to your standards or frameworks like ICH and eCTD.
Generate
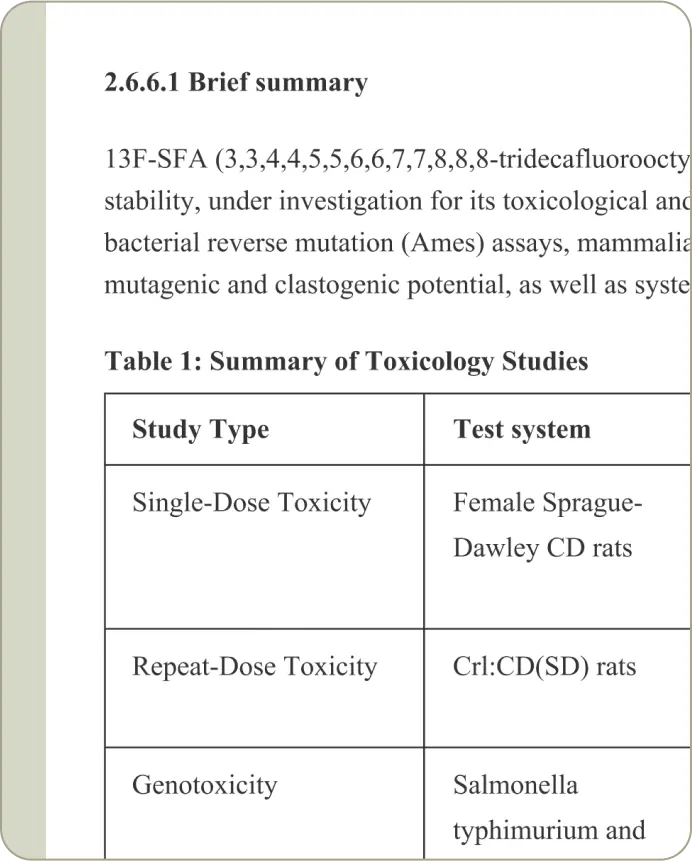
Create high-quality first drafts in minutes with automated text, tables and figures pulled directly from your data.

Collaborate
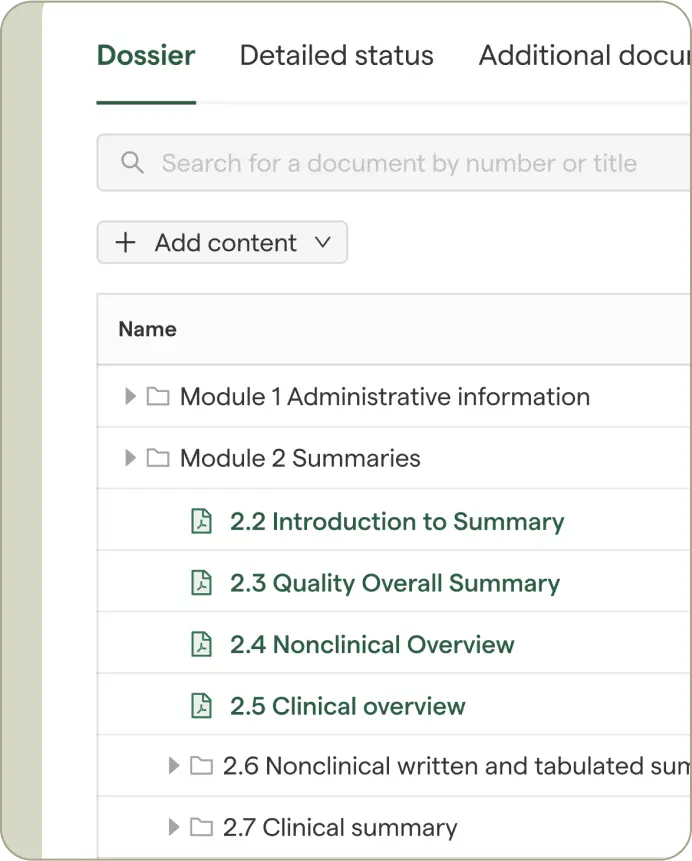
Teams refine content, verify details against source data, and work together in one secure workspace with full traceability.
Submit
Deliver accurate, consistent regulatory dossiers ready for submission.
Why write when you
can weave?
Dossiers require thousands of pages with tons of standard text. Weave generates those in minutes, so you can show people how your newest therapy will change the world.
Preclinical to postmarket
One platform. Every milestone.
Data security & AI privacy
Security and compliance you can count on.
Built with AWS infrastructure, zero data retention and enterprise security controls.
Platform security
architecture
Cloud security:
Built on AWS infrastructure with SOC2 certification launching Q1 2026
Access controls:
Multi-factor authentication, single sign-on support, and role-based permissions
Platform requirements:
No installation headaches—just 4 weeks of onboarding to get up and running from any browser
Integrations:
Secure standalone platform with Veeva integration launching in 2026
Secure AI
processing
Data isolation:
AI models never train on your data with Zero Data Retention agreements
Encrypted processing:
End-to-end encryption protects every interaction and data exchange
Audit trails:
Complete traceability of all AI interactions and data usage in seconds